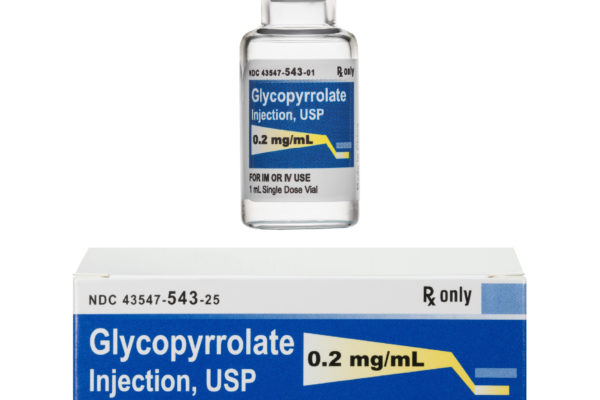
SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR GLYCOPYRROLATE INJECTION, 0.2 mg/mL
CRANBURY, NJ — Solco Healthcare announces its FDA approval for Glycopyrrolate Injection, 0.2mg/mL, which is an AP-rated equivalent to Robinul® by Hikma.
“We are very pleased to have received FDA approval for Glycopyrrolate injection. This approval represents a new chapter for Solco, as we enter into the hospital market with our first injectable product,” said Hai Wang, President of Solco Healthcare. Glycopyrrolate injection is indicated for use as a preoperative antimuscarinic to reduce salivary, tracheobronchial, and pharyngeal secretions; to reduce the volume and free acidity of gastric secretions; and to block cardiac vagal inhibitory reflexes during induction of anesthesia and intubation. According to IQVIA, total market sales for Glycopyrrolate injection for the last twelve months ending January 2019 were $111.7 million.
Solco Healthcare will market Glycopyrrolate injection in 1mL x 25 and 2mL x 25 cartons. Solco anticipates launching this product in the very near future. Please contact your Solco Healthcare National Accounts Director or customer service at 1-888-869-8008 for more information.
