- 700 Atrium Drive, Suite A, Somerset, NJ 08873
- (855) 581-9688
- customerservice@solcohealthcare.com
SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR NEBIVOLOL TABLETS
September 1, 2022 SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR NEBIVOLOL TABLETS SOMERSET, NJ — Solco Healthcare is proud to announce the FDA approval on April 7, 2022 and September 2022 availability of Nebivolol tablets, 2.5mg, 5mg, 10mg and 20mg an AB-rated equivalent to Bystolic® by Allergan, Inc. Nebivolol is a beta-adrenergic blocking agent indicated
SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR TADALAFIL TABLETS
August 11, 2022 SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR TADALAFIL TABLETS SOMERSET, NJ — Solco Healthcare announces its FDA approval and availability of Tadalafil tablets, 2.5mg, 5mg, 10mg and 20mg, which are AB-rated generics equivalent to Cialis® and Adcirca® by Eli Lily. Tadalafil 2.5mg, 5mg, 10mg and 20mg tablets are indicated for the treatment
SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR ENALAPRIL MALEATE TABLETS
July 7, 2022 SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR ENALAPRIL MALEATE TABLETS SOMERSET, NJ — Solco Healthcare announces its FDA approval for Enalapril Maleate tablets, 2.5mg, 5mg, 10mg and 20mg which are AB-rated equivalent to Vasotec® by Bausch Health. “This product was developed by our subsidiary – Prinbury Biopharm for Solco. This approval represents
THE FDA HAS OFFICIALLY REMOVED ZHEJIANG HUAHAI PHARMACEUTICAL FROM IMPORT ALERT
THE FDA HAS OFFICIALLY REMOVED ZHEJIANG HUAHAI PHARMACEUTICAL FROM IMPORT ALERT Somerset, NJ, November 15, 2021 – Solco is pleased to announce that the FDA has lifted the ImportAlert (IA) 66-40, “Detention Without Physical Examination of Drugs From Firms Which Have Not MetDrug GMPs”, Published Date: 11/12/2021, from Zhejiang Huahai Pharmaceutical Co. Ltd., at CoastalIndustrial
SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR AMPHETAMINE SULFATE Tablets, 5mg and 10mg
SOMERSET, NJ — Solco Healthcare announces its FDA approval for Amphetamine Sulfate tablets, 5mg and 10mg, which is an AA-rated equivalent to Evekeo® by Arbor Pharmaceuticals. “This approval represents Solco’s first CII product within the ADHD therapeutic treatment. Amphetamine sulfate tablets will be manufactured in our Prinston Laboratories facility located in Charlotte, NC. We are
SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR GLYCOPYRROLATE INJECTION, 0.2 mg/mL
SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR GLYCOPYRROLATE INJECTION, 0.2 mg/mL CRANBURY, NJ — Solco Healthcare announces its FDA approval for Glycopyrrolate Injection, 0.2mg/mL, which is an AP-rated equivalent to Robinul® by Hikma. “We are very pleased to have received FDA approval for Glycopyrrolate injection. This approval represents a new chapter for Solco, as we enter
Press Release – Update on Valsartan API – A Statement from the Company
FOR IMMEDIATE RELEASE – CRANBURY, NEW JERSEY, August 21, 2018. Huahai US Inc., a subsidiary of Zhejiang Huahai Pharmaceutical Co. Ltd is issuing a statement. Recent articles in several print and on-line media have misrepresented information about the discovery of a trace amount of a genotoxic impurity in Valsartan, an active pharmaceutical ingredient (API) made
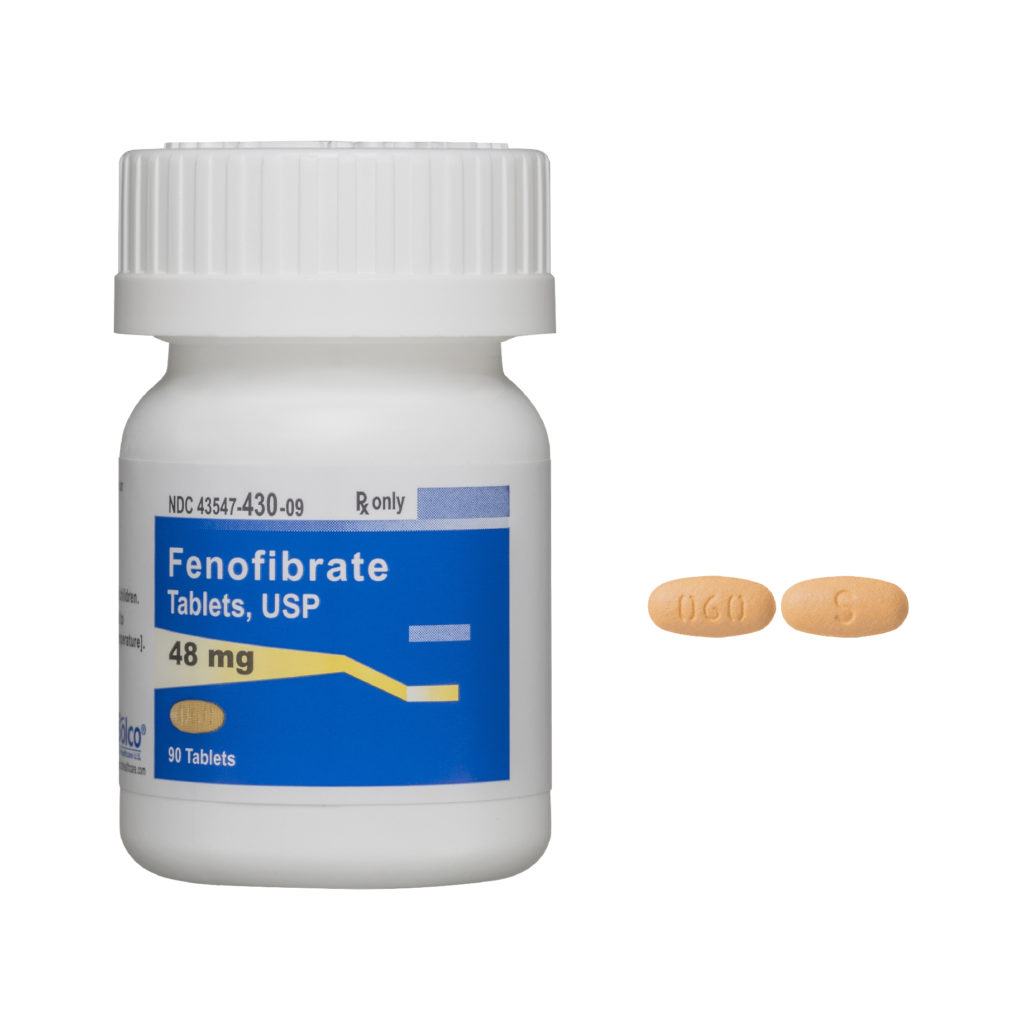
SOLCO HEALTHCARE US ANNOUNCES THE FDA APPROVAL OF FENOFIBRATE TABLETS, 48mg and 145mg
CRANBURY, NJ — Solco Healthcare is proud to announce its FDA approval for Fenofibrate tablets, 48 mg and 145 mg, which is an AB-rated equivalent to Tricor® by Abbvie. Fenofibrate tablets are indicated as adjunctive therapy to diet to reduce elevated low-density lipoprotein cholesterol (LDL-C), total cholesterol (Total-C), Triglycerides and apolipoprotein B (Apo B), and to
SOLCO HEALTHCARE US ANNOUNCES THE LAUNCH OF ENTECAVIR TABLETS
CRANBURY, NJ., April 25, 2018 – Solco Healthcare is proud to announce the launch of Entecavir Tablets, 0.5mg and 1mg, a generic equivalent of Baraclude® Tablets by Bristol-Myers Squibb Company. Solco is offering Entecavir Tablets, 0.5mg and 1mg, in 30-count bottles to its customers. The product is available for shipment immediately. Entecavir tablets are used to treat chronic
SOLCO HEALTHCARE US ANNOUNCES THE LAUNCH OF CLONIDINE HCL ER TABLETS
Cranbury, NJ., April 3, 2018. Solco Healthcare announced today the launch of Clonidine Hydrochloride Extended-Release Tablets, 01.mg, a generic version of Kapvay® Tablets by Concordia Pharmaceuticals Inc. The product is used for the treatment of attention-deficit/hyperactivity disorder (ADHD). The U.S. Food and Drug Administration (FDA) has granted final approval of its Abbreviated New Drug Application for Clonidine
Press Release Archives
-
Call us at 1-855-581-9688
Product Information Contact Us
-
Interested In Partnering
Partner Today Learn More
-
Generic Pharmaceuticals
Our Products View Catalog
About Us
Solco strives to offer patients greater access to affordable medications by providing a broad range of generic prescription products manufactured under the highest quality standards.
- 700 Atrium Dr. Suite A, Somerset, NJ 08873
- SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR NEBIVOLOL TABLETS October 11, 2022
- SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR TADALAFIL TABLETS October 11, 2022
- SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR ENALAPRIL MALEATE TABLETS October 11, 2022
- THE FDA HAS OFFICIALLY REMOVED ZHEJIANG HUAHAI PHARMACEUTICAL FROM IMPORT ALERT November 16, 2021
- SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR AMPHETAMINE SULFATE Tablets, 5mg and 10mg April 17, 2020