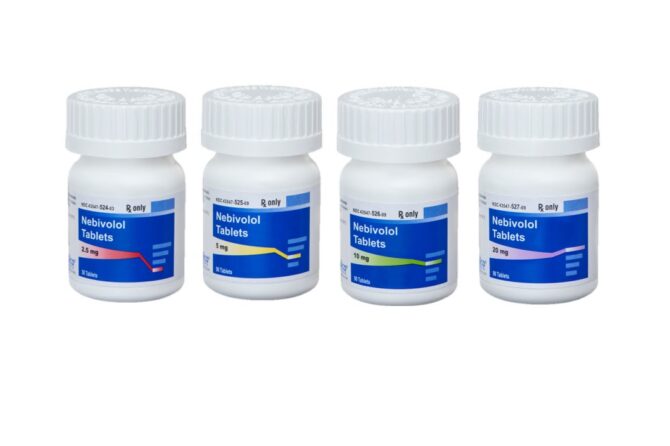
SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR NEBIVOLOL TABLETS
September 1, 2022 SOLCO HEALTHCARE US, RECEIVES FDA APPROVAL FOR NEBIVOLOL TABLETS SOMERSET, NJ — Solco Healthcare is proud to announce the FDA approval on April 7, 2022 and September 2022 availability of Nebivolol tablets, 2.5mg, 5mg, 10mg and 20mg an AB-rated equivalent to Bystolic® by Allergan, Inc. Nebivolol is a beta-adrenergic blocking agent indicated
Read more